3D Printed Nasopharyngeal Swabs For Accelerated COVID-19 Testing
The FAST spiral swab was perfected over 25 iterations in 35 days with clinicians from Stanford Health Care, Harvard-BIDMC and Seattle-area swab clinics.
Speed, Agility and Flexibility to Meet Swab Shortage
A shortage of specialized nasopharyngeal swabs used to collect samples from patients’ noses and throats for diagnosis is one of several bottlenecks in the way of widespread testing. Over the last 35 days, Fathom has been assisting in the development, testing, and production for 3D printed test-kit swabs. Lending our machines and operational expertise, the team at Fathom has worked tirelessly alongside Abiogenix, Harvard University, and HP to ready this life-saving diagnostic tool for use at the front lines of the pandemic.
Using HP’s Multi Jet Fusion Technology To Fight COVID-19 // Read More
Recently validated in a clinical trial at Harvard’s Beth Israel Deaconess Medical Center (BIDMC), the FAST NP Swab was one of 150 designs evaluated and was selected as the most preferred swab on the basis of expert review, collection sufficiency, PCR-compatibility and concordance testing with flocked nasopharyngeal swabs. The FAST NP Swab is FDA Registered: Class 1 Exempt and is available now.
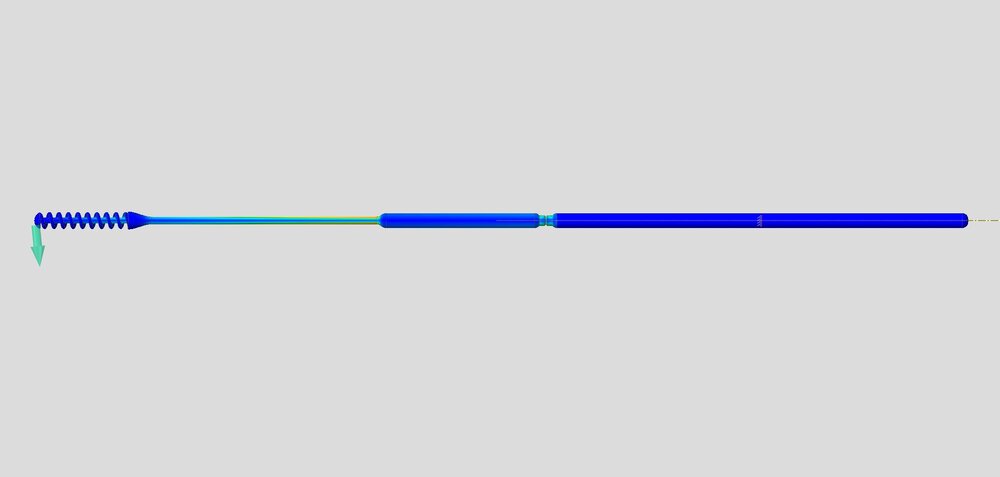
FAST Spiral Nasopharyngeal Swab / / Design Considerations
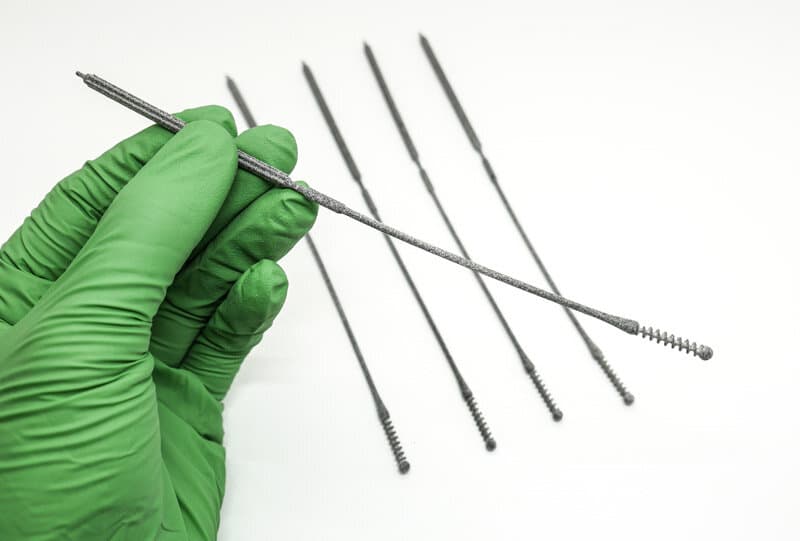
Over 150 NP Swab designs were evaluated in the clinical trial by Harvard-BIDMC. The Abiogenix spiral design, made using HP’s MJF 3D printing technology, was selected as the preferred swab by clinicians and patients.
What were some of the major design considerations that made the FAST design stand out?
Among the design considerations was the importance of the roughly six-inch swabs being rigid enough to reach potentially infected cells at the back of the nasopharynx but flexible enough not to damage soft and sensitive tissues along the way. – Beth Israel Deaconess Medical Center
How did the FAST design stand out?
Working through 25 iterations of the design, the team ultimately came up with a design verified by clinician’s to have the right “feel” of rigidity. One that allows the break point to not be so weak as to buckle when pushed against an obstruction and affords the clinician the ability to perform single-handed breaking, by bending and then twisting the swab to collect the sample.
Although a seemingly simple device at first glance, the entire structure of the swab was redesigned, from the grip and the neck, to the collection spiral, everything underwent countless hours of design, testing/evaluation and redesign to culminate in what is now the final FDA-Registered device. This entire process is a prime example of how additive manufacturing and the right team can facilitate a condensed development life-cycle through a very collaborative and iterative process. Coupled with the extreme motivation to combat a global pandemic, all parties involved were in the right place at the right time to make this concept a reality.
*For initial diagnostic testing for CDC recommends collecting and testing an upper respiratory specimen. Nasopharyngeal specimen is the preferred choice for swab-based testing. Read CDC Article
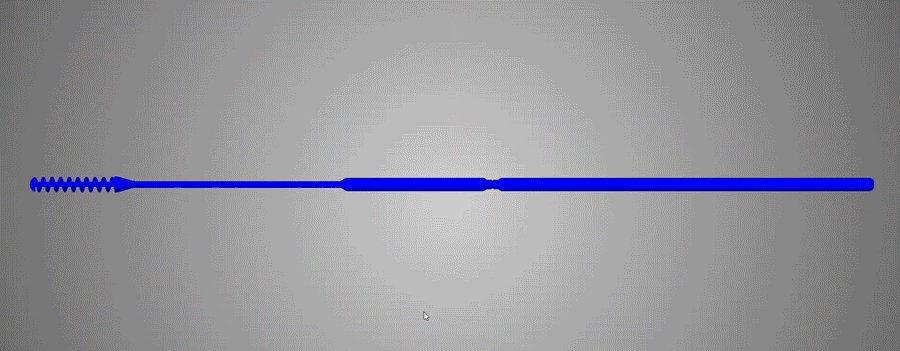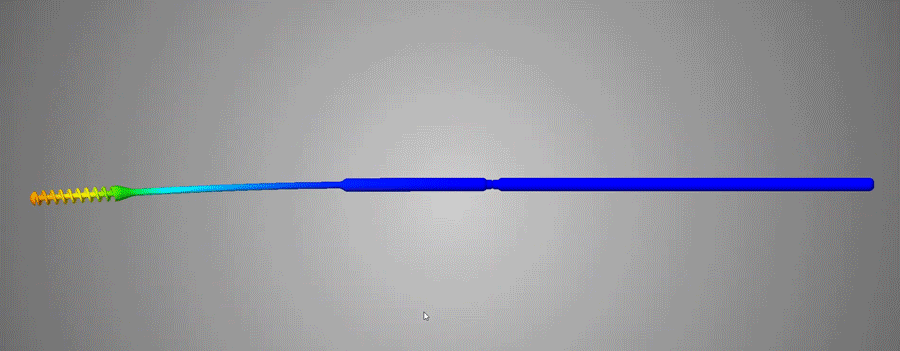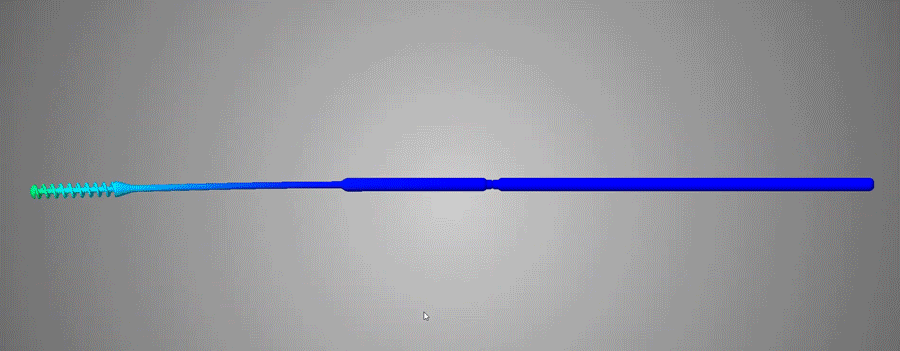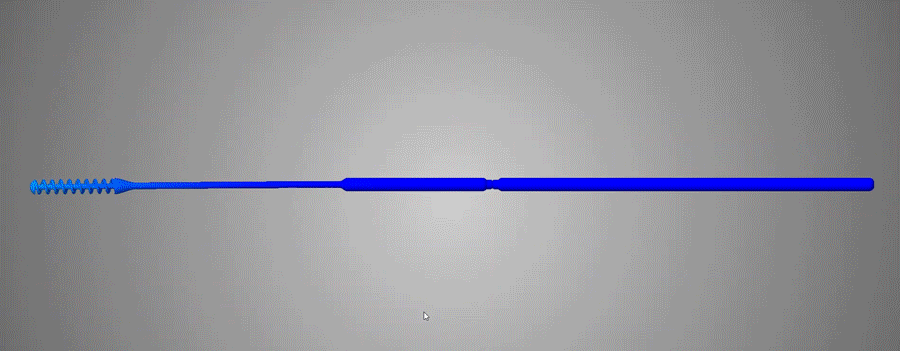
About Abiogenix / /
In March 2020, Dr. Ramy Arnaout from Harvard Medical School put out a call to meet the swab shortage that was limiting COVID-19 testing. We heard the call.
Abiogenix teamed up with Fathom and HP to design and print over 25 iterations of nasopharyngeal swabs.
Working with clinicians on the front-lines, we designed a swab that is easier to use and more comfortable than other 3D printed swabs.
- Designed by Abiogenix
- Refined by clinicians from Stanford Health swab clinic
- Manufactured by Fathom
- Printed using HPs rapid 3D printing technology
- Validated in a clinical trial by Havard-BIDMC
- Tested at Lawrence Livermore National Laboratory
Process Gallery / /








